 Scott Luhmann, MD, a Washington University orthopedic surgeon at St. Louis Children’s Hospital, has expertise in a variety of procedures to treat scoliosis including vertebral body stapling, tethering and resection.
Scott Luhmann, MD, a Washington University orthopedic surgeon at St. Louis Children’s Hospital, has expertise in a variety of procedures to treat scoliosis including vertebral body stapling, tethering and resection.
Here, Dr. Luhmann offers perspective on vertebral body tethering, a promising technique not yet widely used.
Q: What is vertebral body tethering?
A: This technique places a compressive force over the convex side of the spine (slowing down growth) to permit the concave side of the spine to relatively grow more and create a straighter spine. Prior to the introduction of the vertebral body tether, which uses screws placed into the vertebral body, modulating growth of the concave and convex side of the spine was accomplished with staples. These staples were also placed anteriorly, but instead of being placed in the middle of the vertebral body they were placed across the disc spaces between each vertebral body.
Q: What are the advantages of vertebral body tethering over spinal fusion?
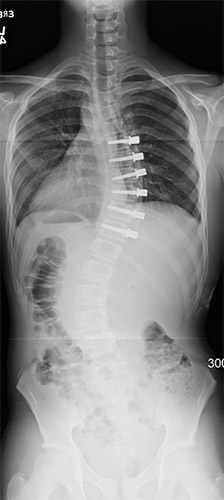 A: Over the past 100 years, significant advancements have been made in spinal fusion to treat scoliosis. These advancements have led to improved fusion rates, lower complication frequency, greater three-dimensional correction of the deformity and more rapid postoperative recovery. However, spine fusions mean fewer motion segments (less spine motion), which may lead to lower function in high-level physical activity (e.g. competitive athletics) and greater chance for spine arthritis. As a result, preservation of spinal motion, particularly in the low back, is an important goal.
A: Over the past 100 years, significant advancements have been made in spinal fusion to treat scoliosis. These advancements have led to improved fusion rates, lower complication frequency, greater three-dimensional correction of the deformity and more rapid postoperative recovery. However, spine fusions mean fewer motion segments (less spine motion), which may lead to lower function in high-level physical activity (e.g. competitive athletics) and greater chance for spine arthritis. As a result, preservation of spinal motion, particularly in the low back, is an important goal.
The desire to maintain spine motion has fueled the development of various growth modulation procedures. The goals of these procedures are to correct the spinal deformity and maintain motion (without fusion). One of these promising techniques that has gained traction in the last 10 years is vertebral body tethering (VBT).
Q: How effective is VBT for scoliosis?
A: Studies on VBT in animal models have demonstrated proof of concept that tethering of the immature spine can alter its growth. The first description of VBT use in humans was a case report in 2010. Early, short-term, single institution studies have been encouraging with few reported serious complications. However, at present there is limited data available on the use of VBT in skeletally immature patients with scoliosis.
There is significant potential for this technology, but little scientific evidence of its efficacy in humans. At present there are no FDA-approved implant systems for scoliosis in the U.S. Spine implants used for VBT are being used in an off-label or unlabeled manner in the U.S.
Q: What are the current indications for VBT?
A: Patients who can benefit from VBT include:
- Skeletally immature patient (Risser 0-3, Sander digital hand score <5). Optimal timing of VBT surgery is necessary to produce a satisfactory spinal alignment at the completion of spinal growth. At present there is insufficient information available to accurately predict when to place a VBT.
- Deformity location: main thoracic
- Idiopathic diagnosis
- Coronal deformity: main thoracic (30-70 degrees), thoracolumbar/lumbar (30-60 degrees)
- Flexibility on side-bending radiographs to less than 30 degrees
- Less than 20 degrees of axial rotation
- Less than 40 degrees of kyphosis
Q: Describe the VBT technique to treat scoliosis.
A: VBT is a thoracoscopic, minimally-invasive technique in which screws are placed into the vertebral bodies on the convex side of the coronal deformity. The screws are placed into the middle of the vertebral body with bicortical purchase under fluoroscopic guidance. A high-strength, braided polypropylene tether is then placed into the screw heads and then sequentially secured to each screw after segmental compression. The technique achieves modest correction of the spinal deformity immediately postoperative.
Q: What are the technical challenges of VBT?
A: There are several. Placement of anterior body screws above T5 and below L4 is not typically possible. There are also questions about the number of vertebra that should be included into the VBT construct, how much tension to place across each vertebral motion segment within the VBT construct, optimal screw trajectory and screw size, placement of VBT across the diaphragm for thoracolumbar curves, and implant prominence.
Q: Is VBT safe for patients?
A: Innovations, especially in the area of surgical spine deformity treatment and advances, typically occur faster than FDA approval timetables. If an innovation isn’t yet FDA-approved, that doesn’t categorically mean it is unsafe or doesn’t work. It simply means there isn’t yet enough medical evidence to prove these devices are safe and efficacious to the FDA, which demands very high level of scientific proof. Until the FDA gives approval, implant systems such as VBT exist in a “grey” area. This can be frustrating to patients and caregivers who are anxious for advances in medicine, yet there is scant medical literature to help them navigate treatment options.
Primum non nocere or “first, to do no harm” is a basic tenet of medicine. This is why for surgical procedures, such as VBT, safety is the pre-eminent concern, even more so than its efficacy or how well it works. If a surgical procedure is safe (infrequent, minor complications, with no significant long-term problems) but only demonstrates mild to moderate efficacy then it may be viewed as a reasonable treatment. However, if the procedure doesn’t have demonstrated, reasonable safety, it is unlikely any level of efficacy will be able to make this a reasonable treatment. This is especially the case for diseases which are not life-threatening, such as scoliosis.
Q: What are some possible complications of VBT?
A: As patients and caregivers consider if VBT is an appropriate treatment, it is important that the potential complications or adverse outcomes are detailed and well-understood as to their likelihood, severity and long-term implications. The list of complications which may occur with VBT are:
- Anesthetic problem (such as allergic reaction or airway problem)
- Injury to the great vessels, heart, lungs
- Uncontrolled bleeding
- Neurologic deficit
- Postoperative pneumothorax
- Surgical site infection
- Screw pullout or symptomatic migration
- Tether breakage
- Failure of VBT to modulate growth
- Over-correction of spinal deformity
- Pleural scarring secondary to surgical approach and presence of screw heads/tether in chest
- Irritation of the diaphragm or psoas due to screws
- Back or chest pain
Q: What are any long-term issues with VBT?
A: The simplistic answer is we don’t know. We do have insight about what happens to the actual tether though.
If we look at other implant systems used in the spine and other bones of the body over the last 50-plus years we can roughly sketch out some possible scenarios for the system currently used for VBT. The fixation in the vertebra are screws that, as a group, have a long history of safety and efficacy. However, the screws used in VBT are designed for use in the posterior spine, and for VBT, they are placed anterior through a minimally invasive or thoracoscopic approach. Based on the collective experience, it appears the screws have the same efficacy and safety profile and few issues with prominence, migration or pullout.
The other aspect of VBT is the tether, which is made of braided polypropylene. This is the workhorse of the system, which compresses across the convex discs and growth plates to modulate spine growth. Since there is no fusion across the vertebral bodies, there will be constant motion on the tether. Like any non-regenerating material that is constantly moving, the tether is subject to fatigue, which can lead to failure or breakage of the tether. It makes sense that the tether will eventually break considering it is implanted in adolescents and will likely be stressed for 60 years or more.
Over the last year, there have been reports of segmental failure of the tether (between two screws), so it is reasonable to assume that in the long-term the tether will likely break in multiple locations. For the sake of the aim of VBT to modulate growth in the immature spine, we only need it to last until the completion of spinal growth. We don’t want the tether to break prior to this time, which would permit the spine deformity to worsen.
Q: What does the tether do to the vertebral bodies, and more importantly, to the disc between the vertebral bodies?
A: The implications of long-term compression of the instrumented disc and the presence of anterior instrumentation in a non-fusion technique is unknown. Changes to the intervertebral discs may occur and, if this happens, may cause axial thoracic back pain or possible disc herniations in the future. Also, it is unknown if increased motion, such as after the tether breaks, through a previously VBT-compressed motion segment is significant. Will this cause back pain? At the present time, we just don’t know.
More research is necessary on VBT safety, timing of VBT placement, VBT tensioning, intervertebral disc health, and long-term patient reported and radiographic outcomes of VBT.









