by Pam McGrath
 St. Louis Children’s Hospital and Washington University School of Medicine have joined a Children’s Oncology Group (COG) clinical trial to help determine the effectiveness of iodine-131 metaiodobenzylguanidine (I-131 MIBG) therapy for children with newly diagnosed high-risk neuroblastoma. Neuroblastoma is the most common solid tumor outside of the brain and spine, and it comprises between 5 and 10 percent of all childhood cancers.
St. Louis Children’s Hospital and Washington University School of Medicine have joined a Children’s Oncology Group (COG) clinical trial to help determine the effectiveness of iodine-131 metaiodobenzylguanidine (I-131 MIBG) therapy for children with newly diagnosed high-risk neuroblastoma. Neuroblastoma is the most common solid tumor outside of the brain and spine, and it comprises between 5 and 10 percent of all childhood cancers.
“We’ve advanced treatments for patients with high-risk neuroblastoma through the optimization of chemotherapy, radiotherapy and surgery, as well as the addition of immunotherapy,” says Washington University pediatric hematologist/oncologist Frederick Huang, MD. “Despite this approach, 40 to 50 percent of patients with high-risk neuroblastoma either do not respond to the established treatments, or they respond initially but experience a relapse within two to three years.”
Certain types of nerve tissues absorb MIBG, including some neuroblastoma cells, and for that reason it has been used to stage this type of cancer within the body for many years. In the COG’s clinical trial, MIBG is combined with I-131, a radioactive iodine, to deliver targeted radiation to the neuroblastoma. After preliminary studies of I-131 MIBG therapy by various institutions, using MIBG therapy for patients with relapsed or refractory high-risk neuroblastoma, the COG began a pilot trial that added I-131 MIBG therapy to the conventional treatments prescribed for children with newly diagnosed high-risk neuroblastoma. Promising outcomes led to the expanded study in which Children’s
Hospital and the School of Medicine are participating.
“We are excited to be the only medical facility in Missouri to offer I-131 MIBG therapy to children. We also are one of only about 20 sites in the country capable of providing this advanced treatment to patients who qualify,” says Dr. Huang. Those criteria include patients having a newly diagnosed high-risk neuroblastoma or a relapsed or refractory neuroblastoma that absorbs MIBG and their being healthy enough to handle the therapy’s side effects.
“Because it is a targeted therapy, I-131 MIBG usually is better tolerated by patients than chemotherapy,” says Daniel Willis, MD, Washington University pediatric hematology/oncology fellow. “The most common side effect is a low blood count due to the bone marrow being temporarily ‘stunned’ by the radiation. As a precaution, we store patients’ stem cells prior to MIBG therapy so that it is available for transplant or infusion should it be needed following treatment.”
Dr. Huang adds, “A less common complication is sinusoidal obstruction syndrome, in which the liver’s blood vessels become inflamed and blocked. Given time, the liver can heal on its own or the disease may be treated with medications.”
I-131 MIBG therapy also is a U.S. Food and Drug Administration-approved treatment for pheochromocytoma and paraganglioma in some pediatric age groups. The new MIBG therapy room at St. Louis Children’s Hospital will facilitate providing treatment for these rare cancers as well.
“Infusion of I-131 MIBG takes just a couple of hours, but the fact that it is a radioactive medicine means special precautions need to be taken for the safety of patients, their caregivers and the hospital staff,” says Dr. Willis.
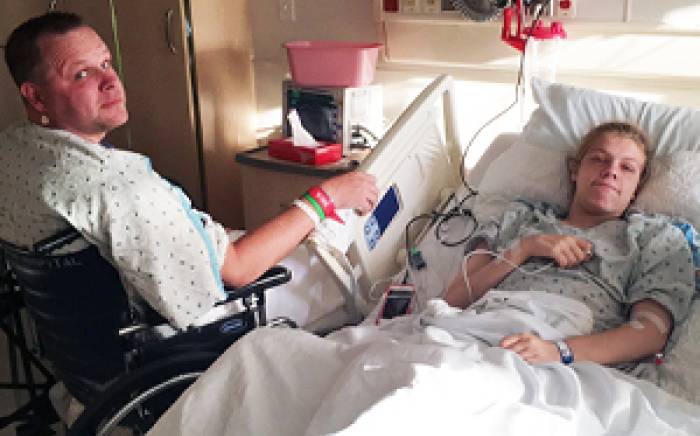
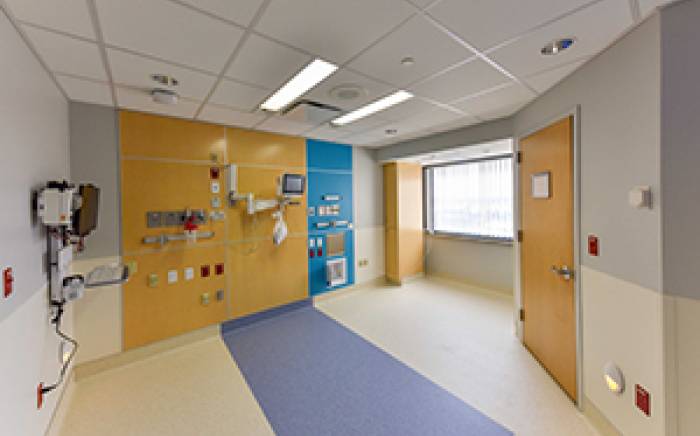
During infusion and for about five days afterward, children  stay in a specially designed, lead-lined patient room on the hospital’s ninth floor. A hotel-like private room and bathroom adjoin it so that parents or other caregivers can comfortably spend their days and nights. Two-way audio and visual communication is always available for caregivers and the patients, and distractions such as television, movies and video games are provided. Other favorite toys that can be easily wiped down and decontaminated may be brought from home.
stay in a specially designed, lead-lined patient room on the hospital’s ninth floor. A hotel-like private room and bathroom adjoin it so that parents or other caregivers can comfortably spend their days and nights. Two-way audio and visual communication is always available for caregivers and the patients, and distractions such as television, movies and video games are provided. Other favorite toys that can be easily wiped down and decontaminated may be brought from home.
“Once patients are released from the room, we take a scan to confirm the drug is targeting the tumor,” says Dr. Willis. “Follow-up scans will help us determine whether or not the tumor is shrinking. In some cases I-131 MIBG therapy may be given again, depending on how the patients tolerated the first treatment and what type of dose they received initially.”
According to Dr. Huang, offering I-131 MIBG therapy is a benefit for patients in the St. Louis region and throughout the Midwest. “Our participation in this new trial truly expands our ability to offer the most advanced therapies for children with high-risk neuroblastoma,” he says.
To learn more about I-131 MIBG therapy or to refer a patient, call Children’s Direct at 800.678.HELP (4357).