The following case study was used by Andrew J. White, MD, associate professor of pediatrics and division director of pediatric rheumatology, Washington University School of Medicine, and director of the St. Louis Children’s Pediatric Residency Program, as part of the “Patient of the Week” (POW) series. Many of the POW case studies cover uncommon illnesses, or common illnesses with unusual symptoms that can be overlooked. If you would like to be added to the POW e-mail distribution, send an email to [email protected].
CC: Newborn with respiratory distress, anemia/thrombocytopenia, abnormal coagulation tests.
HPI: 4 kilogram, 36-week EGA girl, delivered by elective C-section to a 33-year-old G4, P1→2 mother.
Two of the parents’ children died as neonates. In both cases, mother noted absent fetal movement at 37 weeks EGA leading to emergent delivery. The first infant died at age 3 days of intracranial hemorrhage; an incidental note was made of an absent red reflex in one eye. The other died at 1 hour of life with respiratory distress and anemia/thrombocytopenia; autopsy was unrevealing.
Additional FHx: MGM stroke, MGF DVT, PGM stroke. Parents and surviving sibling (5-year-old brother) healthy. Prior maternal evaluation: no setup for neonatal alloimmune thrombocytopenia (mother positive for HPA-1a, the most common antigen responsible for NAIT among Caucasians), low Protein S activity which can be due to pregnancy, low Protein C activity, which was not explainable by pregnancy.
Hospital Course:
Baby developed respiratory distress shortly after delivery. CXR showed nearly complete white out. She was intubated and given surfactant. She was also hypoglycemic in the 20s. She was begun on ampicillin and gentamicin and transported to the SLCH NICU. Despite these measures, her respiratory status worsened, and she was placed on a high-frequency oscillator.
On DOL 1, she had cool, purple lower extremities and oozing from sites of trauma.
- Initial labs: Hb 12.2 g/dl, platelets 93 K/µl, PT 26.2 sec (normal for age 13.1 sec)
- Received fresh frozen plasma, platelets and packed red cell transfusions
Over the next few days, she continued to receive intermittent FFP and platelets. Her PT remained elevated. Her feet had improvement in perfusion; imaging of lower extremities did not show any arterial or venous thrombosis. There was no evidence of sepsis or giant hemangioma as a cause of disseminated intravascular coagulation.
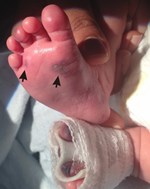 DOL 5: oozing from nose; new ecchymotic lesions of bilateral feet (see image), evolving to skin necrosis; new ecchymoses on scalp, trunk and extremities.
DOL 5: oozing from nose; new ecchymotic lesions of bilateral feet (see image), evolving to skin necrosis; new ecchymoses on scalp, trunk and extremities.
• D-dimer 18.3 (elevated), PT 24.1 sec, platelets 48 K/µl
• Prothrombin = 48%
• Factor VII = 60%
• Factor IX = 51%
• Factor X = 43%
• Fibrinogen = 135 ng/ml
• Protein C < 10% (normal for age = 32%)
• Protein S, free = 56%
• Maternal Protein C = 59% (normal 60-150%)
• Paternal Protein C = 53%
Diagnosis:
- Purpura fulminans, due to homozygous Protein C deficiency
- Ophthalmology exam: right retinal hemorrhage, unable to determine retinal thrombosis; normal exam of left eye
- Head ultrasound: no hemorrhage; absent flow in sagittal sinus consistent with thrombosis
Comments:
Homozygous protein C deficiency is incredibly rare: there are estimated <20 living individuals with this condition in North America. Given the carrier frequency of heterozygous protein C deficiency of 0.2%, the estimated incidence of severe deficiency (typically compound heterozygous) should be 1 per million live births. As illustrated by this family, affected neonates may die without diagnosis.
Protein C is a vitamin K-dependent factor that functions as an anticoagulant following activation by thrombin in the presence of the endothelial cofactor thrombomodulin. Activated Protein C degrades Factors Va and VIIIa, thereby limiting further thrombin generation. The absence of protein C leads to uncontrolled coagulation, manifest as DIC, thrombosis and secondary hemorrhage. In newborns, this clinical picture, termed purpura fulminans, is easily mistaken for sepsis. Affected infants may lack a red reflex if thrombosis occurs in the retina and may be neurologically devastated due to cerebral hemorrhage.
Acute management consists of replacement of Protein C as well as anticoagulation if thrombi are present. In this case, FFP was given every 12 hours initially, followed by Kcentra (a prothrombin complex concentrate product that contains all of the vitamin K-dependent factors) due to the large volume of FFP required. Protein C has the shortest half-life of all the vitamin K-dependent factors at 6-8 hours. A trough Protein C level of >20% was achieved by q 8 hour dosing of Kcentra. Unfractionated heparin was initiated to treat the sagittal sinus thrombosis. A purified Protein C concentrate approved for this condition, Ceprotin, was initiated once available from the manufacturer.
Long-term management consists of a combination of anticoagulation and Protein C replacement. Liver transplantation has been performed in a few patients.
References:
- Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Hemophilia 2008; 14(6):1214-21.
- de Kort EHM, Vrancken SLAG, van Heijst AFJ, et al. Long-term subcutaneous Protein C replacement in neonatal severe Protein C deficiency. Pediatrics 2011; 127(5):e1338-1342.
- Minford A, Behnisch W, Brons P, et al. Subcutaneous protein C concentrate in the management of severe protein C deficiency—experience from 12 centres. British Journal of Haematology 2014; 164: 414-421.